Calculate and Mitigate the Risk of your Pharma Supply Chain
MYTIGATE …
Identifies the most appropriate transport lanes and supply chain partners
Determines the capabilities of supply chain partners
Evaluates and calculates the risks for a given lane and the corresponding supply chain partners
Allocates the information from your temperature data logger to your selected lanes
Uses lane predictive analytics
Is a validated web-based platform according to FDA Guidelines (CFR21 Part 11)
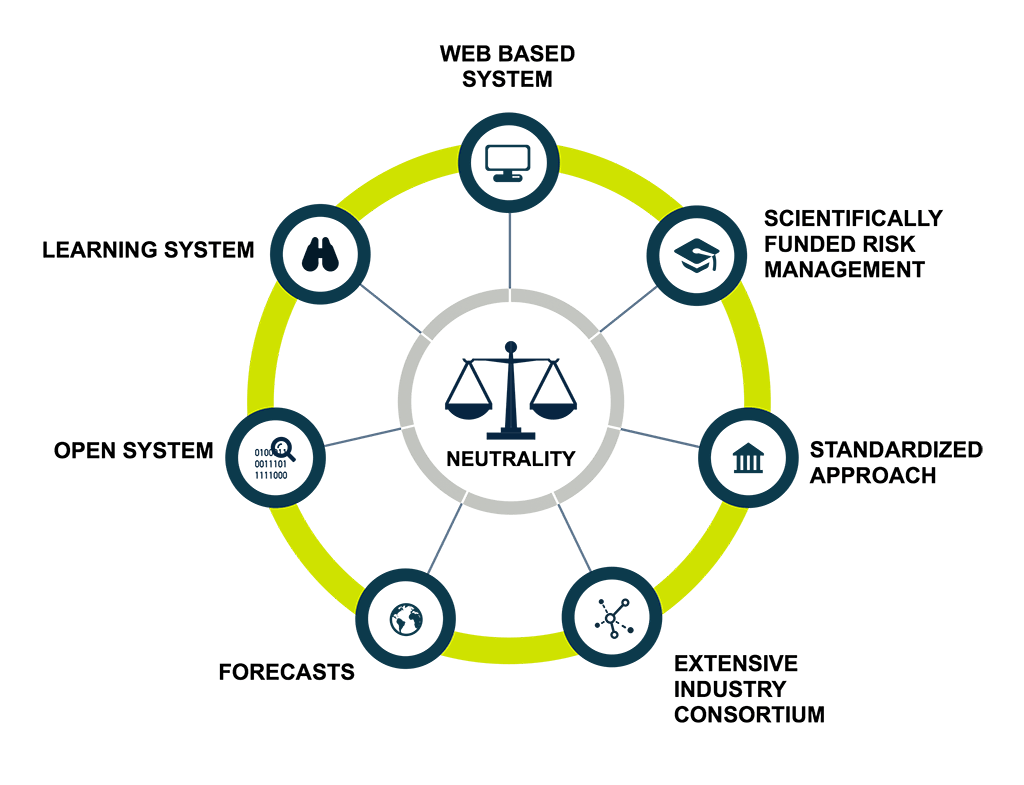
Why Mytigate?
Mytigate is a risk management platform created by and for the pharma Industry with a validated development according to FDA Guidelines (CFR21 Part 11).
We aim to fulfil the market need for a standardized, integrated, preventive supply chain risk management system by:
Providing risk metrics to pharmaceutical producers, wholesalers, forwarders and carriers based on the
capabilities and performance of potential supply chain partners on a specific lane.
Providing a standardized risk analysis and suggestions for risk mitigation measures.
Evaluating which lane and which transport partner will be suitable for a particular product.
Verifying new lanes with regard to regulatory compliance (e.g. EU GDP, FDA, IATA).
Presenting the proper documentation for regulatory authorities.
Being a validated development according to FDA Guidelines (CFR21 Part 11).